Home > Press > Nanoparticle Opens the Door to Clean-Energy Alternatives
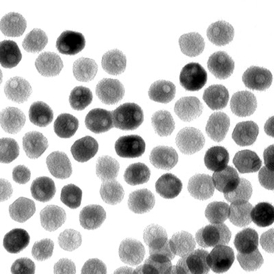 |
| A transmission-electron microscope image of a collection of quasi-spherical nickel phosphide nanoparticles. A team led by Raymond Schaak of Penn State University has found that these nanoparticles can catalyze an important chemical reaction that generates hydrogen from water. Credit: Eric Popczun, Penn State University |
Abstract:
Cheaper clean-energy technologies could be made possible thanks to a new discovery. Led by Raymond Schaak, a professor of chemistry at Penn State University, research team members have found that an important chemical reaction that generates hydrogen from water is effectively triggered -- or catalyzed -- by a nanoparticle composed of nickel and phosphorus, two inexpensive elements that are abundant on Earth. The results of the research will be published in the Journal of the American Chemical Society.
Schaak explained that the purpose of the nickel phosphide nanoparticle is to help produce hydrogen from water, which is a process that is important for many energy-production technologies, including fuel cells and solar cells. "Water is an ideal fuel, because it is cheap and abundant, but we need to be able to extract hydrogen from it," Schaak said. Hydrogen has a high energy density and is a great energy carrier, Schaak explained, but it requires energy to produce. To make its production practical, scientists have been hunting for a way to trigger the required chemical reactions with an inexpensive catalyst. Schaak noted that this feat is accomplished very well by platinum but, because platinum is expensive and relatively rare, he and his team have been searching for alternative materials. "There were some predictions that nickel phosphide might be a good candidate, and we had already been working with nickel phosphide nanoparticles for several years," Schaak said. "It turns out that nanoparticles of nickel phosphide are indeed active for producing hydrogen and are comparable to the best known alternatives to platinum."
To create the nickel phosphide nanoparticles, team members began with metal salts that are commercially available. They then dissolved these salts in solvents, added other chemical ingredients, and heated the solution to allow the nanoparticles to form. The researchers were able create a nanoparticle that was quasi-spherical -- not a perfect sphere, but spherical with many flat, exposed edges. "The small size of the nanoparticles creates a high surface area, and the exposed edges means that a large number of sites are available to catalyze the chemical reaction that produces hydrogen," Schaak explained.
The next step was for team members at the California Institute of Technology to test the nanoparticles' performance in catalyzing the necessary chemical reactions. Led by Nathan S. Lewis, the George L. Argyros Professor of Chemistry at the California Institute of Technology, the researchers performed these tests by placing the nanoparticles onto a sheet of titanium foil and immersing that sheet in a solution of sulfuric acid. Next, the researchers applied a voltage and measured the current produced. They found that, not only were the chemical reactions happening as they had hoped, they also were happening with a high degree of efficacy.
"Nanoparticle technology has already started to open the door to cheaper and cleaner energy that is also efficient and useful," Schaak said. "The goal now is to further improve the performance of these nanoparticles and to understand what makes them function the way they do. Also, our team members believe that our success with nickel phosphide can pave the way toward the discovery of other new catalysts that also are comprised of Earth-abundant materials. Insights from this discovery may lead to even better catalysts in the future."
In addition to Schaak and Lewis, other researchers who contributed to this study include Eric J. Popczun, Carlos G. Read, Adam J. Biacchi, and Alex M. Wiltrout from Penn State; and James R. McKone from the California Institute of Technology.
The research was funded by the U.S. National Science Foundation and the U.S. Department of Energy. The team has filed a patent application.
####
For more information, please click here
Contacts:
Raymond Schaak:
814-865-8600
Barbara Kennedy (PIO)
814-863-4682
Copyright ? Penn State
If you have a comment, please Contact us.Issuers of news releases, not 7th Wave, Inc. or Nanotechnology Now, are solely responsible for the accuracy of the content.
Bookmark:








News and information
![]() Nanoparticles helping to recover more oil June 15th, 2013
Nanoparticles helping to recover more oil June 15th, 2013
![]() Monell-led research identifies scent of melanoma: New research may lead to early non-invasive detection and diagnosis June 14th, 2013
Monell-led research identifies scent of melanoma: New research may lead to early non-invasive detection and diagnosis June 14th, 2013
![]() Journal Nanotechnology Progress International (JONPI): New issue coming out very soon! Watch for it! June 14th, 2013
Journal Nanotechnology Progress International (JONPI): New issue coming out very soon! Watch for it! June 14th, 2013
![]() Controlling magnetic clouds in graphene June 14th, 2013
Controlling magnetic clouds in graphene June 14th, 2013
Chemistry
![]() Polymer structures serve as 'nanoreactors' for nanocrystals with uniform sizes, shapes: Tiny chemistry June 11th, 2013
Polymer structures serve as 'nanoreactors' for nanocrystals with uniform sizes, shapes: Tiny chemistry June 11th, 2013
![]() UC Santa Barbara study provides a new framework for understanding the energetics of ionic liquids June 8th, 2013
UC Santa Barbara study provides a new framework for understanding the energetics of ionic liquids June 8th, 2013
![]() Whispering light hears liquids talk: University of Illinois researchers build first-ever bridge between optomechanics and microfluidics June 7th, 2013
Whispering light hears liquids talk: University of Illinois researchers build first-ever bridge between optomechanics and microfluidics June 7th, 2013
![]() Re-creating the original colors of treasured ivory carvings from the ancient past May 29th, 2013
Re-creating the original colors of treasured ivory carvings from the ancient past May 29th, 2013
Govt.-Legislation/Regulation/Funding/Policy
![]() Discovery of new material state counterintuitive to laws of physics June 14th, 2013
Discovery of new material state counterintuitive to laws of physics June 14th, 2013
![]() Nanobiotix receives approval from ANSM to start new Clinical Trial with Lead Product NBTXR3: Second indication for NanoXray product in Head and Neck cancer patients June 14th, 2013
Nanobiotix receives approval from ANSM to start new Clinical Trial with Lead Product NBTXR3: Second indication for NanoXray product in Head and Neck cancer patients June 14th, 2013
![]() Researchers gain new molecular-level understanding of the brain's recovery after stroke June 13th, 2013
Researchers gain new molecular-level understanding of the brain's recovery after stroke June 13th, 2013
![]() Unzipped nanotubes unlock potential for batteries: Rice University lab combines graphene nanoribbons with tin oxide for improved anodes June 13th, 2013
Unzipped nanotubes unlock potential for batteries: Rice University lab combines graphene nanoribbons with tin oxide for improved anodes June 13th, 2013
Discoveries
![]() Nanoparticles helping to recover more oil June 15th, 2013
Nanoparticles helping to recover more oil June 15th, 2013
![]() Discovery of new material state counterintuitive to laws of physics June 14th, 2013
Discovery of new material state counterintuitive to laws of physics June 14th, 2013
![]() Closer to reality June 14th, 2013
Closer to reality June 14th, 2013
![]() Imec showcases innovation in RRAM R&D at VLSI Technology Symposium June 14th, 2013
Imec showcases innovation in RRAM R&D at VLSI Technology Symposium June 14th, 2013
Announcements
![]() Nanoparticles helping to recover more oil June 15th, 2013
Nanoparticles helping to recover more oil June 15th, 2013
![]() Imec showcases innovation in RRAM R&D at VLSI Technology Symposium June 14th, 2013
Imec showcases innovation in RRAM R&D at VLSI Technology Symposium June 14th, 2013
![]() Nanobiotix receives approval from ANSM to start new Clinical Trial with Lead Product NBTXR3: Second indication for NanoXray product in Head and Neck cancer patients June 14th, 2013
Nanobiotix receives approval from ANSM to start new Clinical Trial with Lead Product NBTXR3: Second indication for NanoXray product in Head and Neck cancer patients June 14th, 2013
![]() Journal Nanotechnology Progress International (JONPI): New issue coming out very soon! Watch for it! June 14th, 2013
Journal Nanotechnology Progress International (JONPI): New issue coming out very soon! Watch for it! June 14th, 2013
Interviews/Book Reviews/Essays/Reports/Podcasts/Journals
![]() Closer to reality June 14th, 2013
Closer to reality June 14th, 2013
![]() Journal Nanotechnology Progress International (JONPI): New issue coming out very soon! Watch for it! June 14th, 2013
Journal Nanotechnology Progress International (JONPI): New issue coming out very soon! Watch for it! June 14th, 2013
![]() Controlling magnetic clouds in graphene June 14th, 2013
Controlling magnetic clouds in graphene June 14th, 2013
![]() Nano-thermometer enables first atomic-scale heat transfer measurements June 13th, 2013
Nano-thermometer enables first atomic-scale heat transfer measurements June 13th, 2013
Patents/IP/Tech Transfer/Licensing
![]() DRYWIRED? Receives MILITARY-STANDARD Certifications from QUANTA LABORATORIES for Its Latest Protective Nano-Coating Technology June 5th, 2013
DRYWIRED? Receives MILITARY-STANDARD Certifications from QUANTA LABORATORIES for Its Latest Protective Nano-Coating Technology June 5th, 2013
![]() Apple patents point to slimmer battery tech June 1st, 2013
Apple patents point to slimmer battery tech June 1st, 2013
![]() Patent for Coaxial Nanofibre Production: Scientists from Contipro have patented new jet for effective coaxial nanofibers production May 31st, 2013
Patent for Coaxial Nanofibre Production: Scientists from Contipro have patented new jet for effective coaxial nanofibers production May 31st, 2013
![]() Element Six Acquires the Assets and Intellectual Property of Group4 Labs, Inc to Expand Portfolio of Synthetic Diamond Materials for the Semiconductor Industry May 31st, 2013
Element Six Acquires the Assets and Intellectual Property of Group4 Labs, Inc to Expand Portfolio of Synthetic Diamond Materials for the Semiconductor Industry May 31st, 2013
Energy
![]() Nanoparticles helping to recover more oil June 15th, 2013
Nanoparticles helping to recover more oil June 15th, 2013
![]() Nano-thermometer enables first atomic-scale heat transfer measurements June 13th, 2013
Nano-thermometer enables first atomic-scale heat transfer measurements June 13th, 2013
![]() Filmmaking magic with polymers June 12th, 2013
Filmmaking magic with polymers June 12th, 2013
![]() Further research on effects of nanomaterials: BASF participates in BMBF research project on safety of nanomaterials: Results allow easier and faster evaluation of nanoparticle behavior June 12th, 2013
Further research on effects of nanomaterials: BASF participates in BMBF research project on safety of nanomaterials: Results allow easier and faster evaluation of nanoparticle behavior June 12th, 2013
Water
![]() Discovery of new material state counterintuitive to laws of physics June 14th, 2013
Discovery of new material state counterintuitive to laws of physics June 14th, 2013
![]() Filmmaking magic with polymers June 12th, 2013
Filmmaking magic with polymers June 12th, 2013
![]() Iran Applying Nanotechnology in Growing Number of Industries June 9th, 2013
Iran Applying Nanotechnology in Growing Number of Industries June 9th, 2013
![]() Researchers enlist Cameca Nanosims Microprobe to determine origins of lunar water: Microprobes's results indicate water on Earth and Moon has similar origin June 4th, 2013
Researchers enlist Cameca Nanosims Microprobe to determine origins of lunar water: Microprobes's results indicate water on Earth and Moon has similar origin June 4th, 2013
Source: http://www.nanotech-now.com/news.cgi?story_id=47648
columbine Newton virginia tech shooting China glock 121212 Concert Columbine shooting
কোন মন্তব্য নেই:
একটি মন্তব্য পোস্ট করুন